Research Description
|
Overview
|
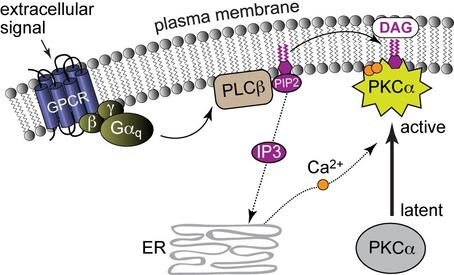
Protein Kinase C as a paradigm of coincidence detection and signal transduction at the membrane surface
Protein kinases are key regulatory enzymes that catalyze phosphorylation of their target proteins. Of the 518 known human kinases, 63 belong to the AGC kinase subfamily. Protein Kinase C (PKC) isoenzymes are lipid-activated AGC kinases that regulate vital cellular processes, such as gene expression, migration, proliferation, and apoptosis, through phosphorylation of their targets. Recent demonstrations of the tumor-suppressing roles of PKCs in cancer and their involvement in progression of cardiovascular and neurodegenerative diseases underscore the need to understand precisely how PKCs are regulated. Despite the relevance of PKC in human health and progress in synthesis of PKC agonists, the structural basis for how the cellular activities of PKCs (and of AGC kinases in general) is controlled remains poorly understood. The activities and sub-cellular localizations of AGC kinases are determined by protein domains that are distinct from the kinase catalytic core.
Through the focus on those domains, their lipid- and metal-ion sensing functions, and interactions with protein partners we aim to uncover the mechanism of PKC activation and develop a broad structural biology picture of protein-lipid recognition events that underlie numerous signal transduction and membrane trafficking processes.
Through the focus on those domains, their lipid- and metal-ion sensing functions, and interactions with protein partners we aim to uncover the mechanism of PKC activation and develop a broad structural biology picture of protein-lipid recognition events that underlie numerous signal transduction and membrane trafficking processes.
Multi-modular proteins and the need for integrative structural biology approaches
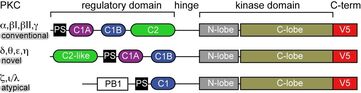 Multimodular architecture of PKCs.
Multimodular architecture of PKCs.
Many proteins involved in signal transduction share the multi-modular architecture of PKCs, where flexible linker regions connect individual protein domains. The linkers ensure conformational plasticity of the signaling proteins and enable them to interact with a variety of protein partners and membrane-embedded ligands. The individual protein domains, each having a highly specialized function, enable a complex and nuanced response to different stimuli generated in the cell. X-ray crystallography provides high-resolution crystal structures of the individual protein domains in the apo- forms and bound to soluble ligands. However, crystalline picture does not adequately capture the dynamic landscape of highly flexible protein systems, and oftentimes incorporation of lipid ligands and low-affinity ligands is difficult. Here, Nuclear Magnetic Resonance (NMR) spectroscopy offers unique advantages through its ability to probe protein-membrane interactions, and the dynamics at both intra- and inter-domain levels. In addition, atomistic molecular dynamics (MD) simulations can provide information on the discrete steps and conformational changes involved in membrane recruitment and recognition of membrane-embedded ligands.
Overall, integrative structural biology approaches augmented by other biochemical and biophysical techniques are required to develop a comprehensive picture of signal transduction processes at the membrane surface.
Overall, integrative structural biology approaches augmented by other biochemical and biophysical techniques are required to develop a comprehensive picture of signal transduction processes at the membrane surface.
Current projects
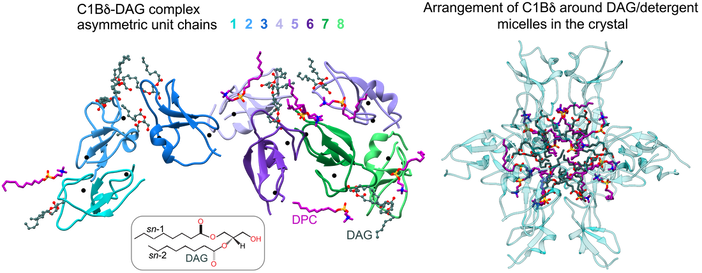 Crystal structure of the C1-DAG complex
Crystal structure of the C1-DAG complex
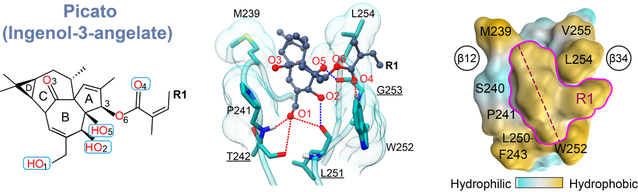
1. Mechanism of diacylglycerol (DAG) sensing by the C1 domains
Diacylglycerol (DAG) is an essential lipid whose 1,2-sn-stereoisomer serves both as second messenger in signal transduction pathways and metabolic precursor. The impressive diversity of DAG signaling output is mediated via its interactions with seven families of effector proteins, including PKC isoenzymes, that execute broad sets of regulatory functions. The stereospecific recognition of DAG is mediated by specialized Zn finger modules called conserved homology-1 (C1) domains. How C1 domains recognize and capture DAG remained an unresolved question in the lipid signaling field for nearly four decades. Recently, our laboratory overcame the challenges associated with extreme hydrophobicity of the C1 complexes and obtained the very first crystal structure of DAG complexed C1 domain.
The ongoing work is aimed at combining those structural insights with MD simulations and NMR studies, to arrive at a comprehensive mechanistic picture of DAG recognition in lipid membranes. Current efforts are also directed at the implementation of new membrane mimics for NMR studies, with the objective to replicate the native DAG-enriched membrane microenvironments sensed by C1 domains. Intimately related to the DAG sensing mechanism is the question of differential sensitivity of C1 domains to DAG, amply illustrated by the tandem C1A and C1B domains of PKC isoforms. What structural features and biophysical properties of C1s make them high- versus low-affinity DAG sensors? Are C1 domains capable of sensing lipid ligands other than DAG? We are actively exploring these research directions that can potentially reveal novel mechanisms of coincidence detection of lipid signals. The scope of this project extends beyond PKC, as C1 domains are ubiquitous protein modules. Understanding the molecular basis of lipid specificities will lead to the development of comprehensive structural views of multi-modular DAG effector proteins at the membrane interface.
2. Exogenous PKC agonists: mechanisms of action and opportunities for drug design
The approach of targeting C1 domains of PKC for pharmaceutical modulation shows considerable promise for the treatment cancers, neurodegenerative disorders, and HIV/AIDS. To that end, rational design of potent C1 modulators requires detailed information regarding: (i) functional properties of the ligand binding site; (ii) key residues that participate in ligand recognition and capture; (iii) role of binding site dynamics in ligand interactions; and (iv) role of other signaling lipids in the formation of C1-ligand complexes. Our laboratory has recently developed a methodology to crystallize C1 domains complexed to the potent PKC ligands. We are currently applying this methodology, in combination with NMR spectroscopy and MD simulations, to determine the structural basis of C1-agonist-membrane interactions for a chemically diverse set of exogenous PKC agonists. This information will provide the foundation for the design of lead compounds that target PKC and other DAG effector proteins.
3. Xenobiotic metal ions as probes of protein structure and dynamics
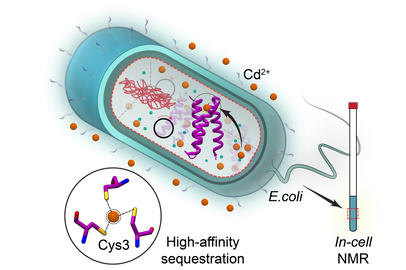 In-cell NMR to probe metal interactions in E.coli
In-cell NMR to probe metal interactions in E.coli
In human biology, xenobiotic (or non-nutritive) metal ions are perceived as foes rather than friends. Their capacity for ionic mimicry enables them to compete with nutritive metal ions for binding sites in biological macromolecules and disrupt vital cellular functions. We discovered that some xenobiotic metal ions, such as Pb(II) and the members of the lanthanide series have unique physicochemical properties with respect to their interactions with oxygen-rich Ca(II)-binding sites of C2 domains, a class of Ca(II)-dependent peripheral membrane modules.
Using an experimental approach that integrates NMR, EPR, and quantum chemical calculations, we seek to understand the regulatory role of inter-domain interactions in Ca(II)-dependent proteins (Synaptotagmin I and PKC) and uncover the structural basis of their C2-driven recruitment to lipid membranes. These studies are possible only because xenobiotic metal ions enable us to generate metal ion-ligated protein states that are inaccessible through the use of native Ca(II). In addition to the in-vitro work, we are applying in-cell NMR approaches to probe metal-ion interactions with C2 domains and other proteins in the crowded environment.
Using an experimental approach that integrates NMR, EPR, and quantum chemical calculations, we seek to understand the regulatory role of inter-domain interactions in Ca(II)-dependent proteins (Synaptotagmin I and PKC) and uncover the structural basis of their C2-driven recruitment to lipid membranes. These studies are possible only because xenobiotic metal ions enable us to generate metal ion-ligated protein states that are inaccessible through the use of native Ca(II). In addition to the in-vitro work, we are applying in-cell NMR approaches to probe metal-ion interactions with C2 domains and other proteins in the crowded environment.
Links to the research featured on the cover of journal Metallomics:
http://xlink.rsc.org/?DOI=C8MT00135A
http://xlink.rsc.org/?DOI=D0MT00002G
Link to the research featured on the cover of journal Biochemistry:
https://pubs.acs.org/doi/10.1021/acs.biochem.8b01235
http://xlink.rsc.org/?DOI=C8MT00135A
http://xlink.rsc.org/?DOI=D0MT00002G
Link to the research featured on the cover of journal Biochemistry:
https://pubs.acs.org/doi/10.1021/acs.biochem.8b01235
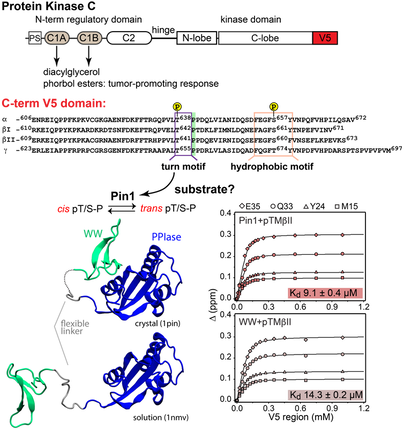
4. Pin1 and its role in AGC kinase regulation
Pin1 is a peptidyl-prolyl isomerase that catalyzes the cis-trans isomerization of pThr/pSer-Pro bonds in its target proteins. Moreover, Pin1 regulates the activities of proteins involved in cancer initiation and progression, and Pin1 is overexpressed in various cancers. Thus, development of Pin1 inhibitors is a focus for both pharmaceutical companies and academic research groups. Recent in-cell studies show that Pin1 interacts with, and regulates, the stabilities of at least six AGC kinases: PKC, Akt, S6K, SGK, GRK2, and LATS. The molecular mechanisms that underlie regulation are not well understood.
Our current research efforts are directed towards uncovering the structural basis of Pin1-AGC kinase interactions, developing sensitive methods for detection of Pin1 interactions with therapeutic agents, and establishing molecular mechanisms of Pin1 dysregulation in disease through the focus on the cancer-related Pin1 variants.
Pin1 is a peptidyl-prolyl isomerase that catalyzes the cis-trans isomerization of pThr/pSer-Pro bonds in its target proteins. Moreover, Pin1 regulates the activities of proteins involved in cancer initiation and progression, and Pin1 is overexpressed in various cancers. Thus, development of Pin1 inhibitors is a focus for both pharmaceutical companies and academic research groups. Recent in-cell studies show that Pin1 interacts with, and regulates, the stabilities of at least six AGC kinases: PKC, Akt, S6K, SGK, GRK2, and LATS. The molecular mechanisms that underlie regulation are not well understood.
Our current research efforts are directed towards uncovering the structural basis of Pin1-AGC kinase interactions, developing sensitive methods for detection of Pin1 interactions with therapeutic agents, and establishing molecular mechanisms of Pin1 dysregulation in disease through the focus on the cancer-related Pin1 variants.
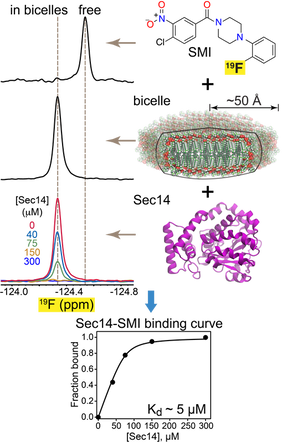 F-19 NMR-based approach to characterize Sec14 interaction with small-molecule inhibitor (SMI).
F-19 NMR-based approach to characterize Sec14 interaction with small-molecule inhibitor (SMI).
5. Mechanisms of lipid exchange processes mediated by PITPs
Phosphatidylinositol/phosphatidylcholine exchange proteins (PITPs) are key players in the inositol lipid-based phosphoinositide signaling pathways. Through their lipid-exchange function, PITPs integrate lipid metabolism with phosphatidylinositol signaling. Studies conducted in S. cerevisiae demonstrate that Sec14 PITP is an attractive target for the development of anti-fungal therapies.
In collaboration with the Bankaitis laboratory at Texas A&M, we are applying integrative structural biology approaches to understand the mechanisms of the homo- and heterotypic lipid exchange reactions and their inhibition by the antifungal compounds. The scope and focus of our work demand development of innovative experimental approaches to monitor the lipid exchange processes, and the binding of small molecule inhibitors to the PITPs. One of the recent additions to our repertoire of biophysical techniques is solution 19F NMR spectroscopy that offers important advantages, such as high-sensitivity and adaptability to high-throughput formats, compared to conventional spectroscopic methods.
Phosphatidylinositol/phosphatidylcholine exchange proteins (PITPs) are key players in the inositol lipid-based phosphoinositide signaling pathways. Through their lipid-exchange function, PITPs integrate lipid metabolism with phosphatidylinositol signaling. Studies conducted in S. cerevisiae demonstrate that Sec14 PITP is an attractive target for the development of anti-fungal therapies.
In collaboration with the Bankaitis laboratory at Texas A&M, we are applying integrative structural biology approaches to understand the mechanisms of the homo- and heterotypic lipid exchange reactions and their inhibition by the antifungal compounds. The scope and focus of our work demand development of innovative experimental approaches to monitor the lipid exchange processes, and the binding of small molecule inhibitors to the PITPs. One of the recent additions to our repertoire of biophysical techniques is solution 19F NMR spectroscopy that offers important advantages, such as high-sensitivity and adaptability to high-throughput formats, compared to conventional spectroscopic methods.